About the Study
The VALOR Study is being done to see if an investigational medicine can be safely given to infants, children, and teenagers and if it may help improve their heart failure.

Who can join the VALOR Study?
- There will be about 342 participants from around the world.
- Children with heart failure who are between the ages of 29 days to 17 years (before 18th birthday) may be eligible to join the study.
- There are other requirements to join the study. Your child’s cardiologist can help you decide if your child may be eligible.
What happens during the VALOR Study?
The study lasts about 1 year. It has three parts. In total, there will be about 12 scheduled visits to the study clinic.

Screening
You’ll be given an Informed Consent Form that contains information about the study. Your child may also be given an Informed Assent Form. Make sure you understand the study and ask any questions you may have. Signing these forms means you agree to join the study. Your child will have health checks and tests to see if they qualify for the study. You and your child can choose to leave the study at any time.
- Informed Consent/ Informed Assent
- Health Checks and Tests
- Confirm Eligibility

Study Treatment
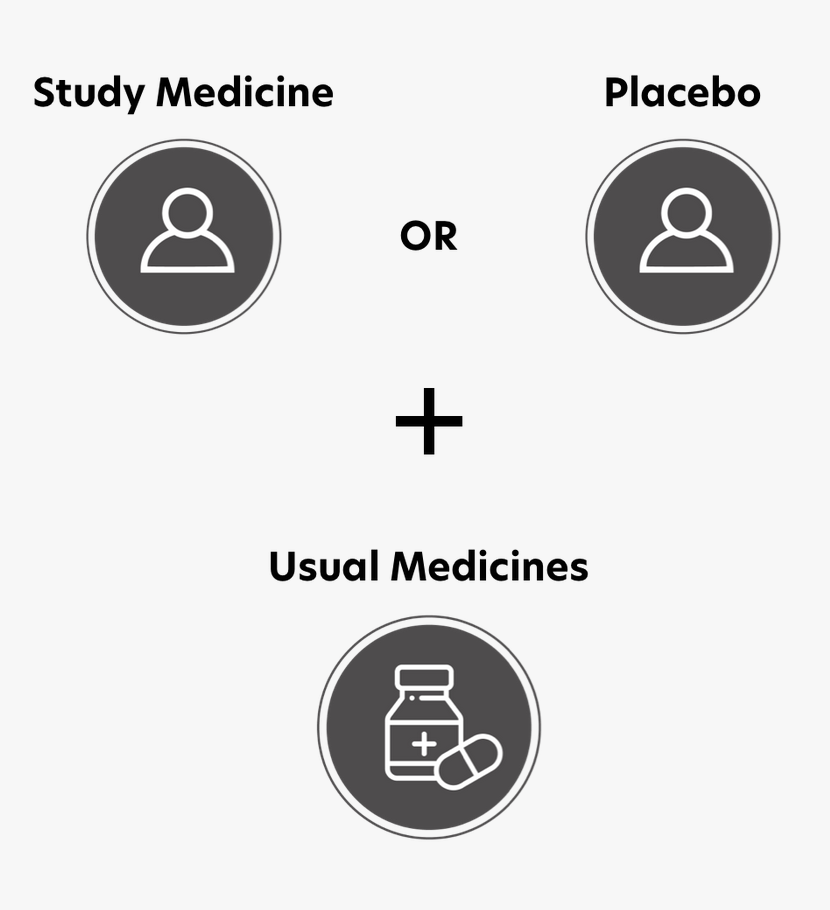
Your child will take the study medicine and have health checks and tests according to a schedule. The study medicine could be vericiguat or a placebo. This study is testing vericiguat (MK-1242) in children and teenagers with a certain type of heart failure. In this type of heart failure, the heart does not pump as well as it should. Vericiguat is experimental in children and teenagers. It has not been approved for heart failure in patients under the age of 18. The placebo looks just like the study drug, but has no active ingredients. Your child will continue to take their usual medicines and keep seeing their usual cardiologist.

Follow-up
There is one final telephone call for a health check after your child is done taking the study medicine.
What happens at study visits?
Your child will have health checks and tests at study visits. The study doctor will also talk to you and your child and ask questions. The information will help the researchers understand if the study medicine may safely help children with heart failure.
You’ll be familiar with most of the tests. Some tests will only be done a few times during the study.
For example, after screening is complete and your child enters the study:
Blood Tests
Blood samples will be taken at approximately 7 visits.

Heart Activity
An ECG heart test will be done at 5 visits.
Physical Exam
A complete physical exam will be done at the first and final clinic visits.
What is the study medicine?
The study medicine, vericiguat, is being compared to a placebo. The placebo looks just like vericiguat, but has no active ingredients.
The study medicine comes as a liquid or tablet to be taken once a day with food. Whether your child gets the liquid or tablet will depend on their weight. The medicine should be taken approximately at the same time each day (about every 24 hours). Your child will continue to take their usual medicines as well.
Study Locations
Locations shown may have changed in some cases. Please call the number listed in the location results to confirm the nearest study site. Talk with a study site member for more information.
Frequently Asked Questions
General Study Questions
Talk to your child’s cardiologist about the VALOR Study. They’ll be able to answer your questions or reach out to a VALOR study doctor to find out more.
There are risks and benefits to being in any clinical study, which a study doctor can discuss with you in greater detail. The study doctor and study team will answer any questions you may have and review eligibility criteria to see if your child may be eligible to participate.
No. Your child will continue to take their usual medicines.
Your child must be less than 18 years old at the time you sign the Informed Consent Form (the document that shows you agree to your child participating in the study). A child who turns 18 during their participation in the study can remain in the study.
Understanding clinical studies is important when making a decision about joining one. Our FAQs have answers to common questions for parents and children. See more at the link below.
What can you do next?
If you are interested in your child taking part in this clinical study, please take the next step to see if your child may be eligible.
Discuss with your child’s doctor or care team
Print this page with details about the study or email it to your child’s doctor to discuss the clinical study during your child’s next visit.
Get help talking with your child’s doctor or care team
Contact our Study Information Center
To learn more, call 1-888-577-8839.
NATIONAL TRIAL REFERENCE NUMBER
NCT05714085
When speaking to your doctor or clinical trial representative, please have the trial reference number available.
Taking part in a clinical study is an important decision
Deciding to allow your child to take part in a clinical study is an important decision.
If you are considering whether your child will join a clinical study, learn as much as you can about:
- The investigational treatment that is being studied
- What the possible risks and benefits are for your child
Talk to your child’s doctor about the clinical study before you decide whether they will join.
Read our “What to Consider” page for more questions to ask and think about

